Types of Water in the Pharmaceutical Industry: Water is the most widely used raw material in the pharmaceutical industry. It plays a critical role as a solvent, cleaning agent, ingredient, and processing aid. Because water can easily carry contaminants—chemical, microbial, or endotoxins—its quality must strictly comply with pharmacopeial standards such as USP, EP, and IP.
Water is the most widely used raw material in the pharmaceutical industry. It plays a critical role as a solvent, cleaning agent, ingredient, and processing aid. Because water can easily carry contaminants—chemical, microbial, or endotoxins—its quality must strictly comply with pharmacopeial standards such as USP, EP, and IP.
This article explores the types of pharmaceutical water, their uses, and the techniques used for their preparation.
Why Water Quality Matters in Pharmaceuticals
Pharmaceutical water must be:
-
Chemically pure
-
Microbiologically safe
-
Free from pyrogens (endotoxins)
-
Consistent in quality
Poor water quality can lead to:
-
Product contamination
-
Reduced drug stability
-
Regulatory non-compliance
-
Patient safety risks
Classification of Pharmaceutical Water
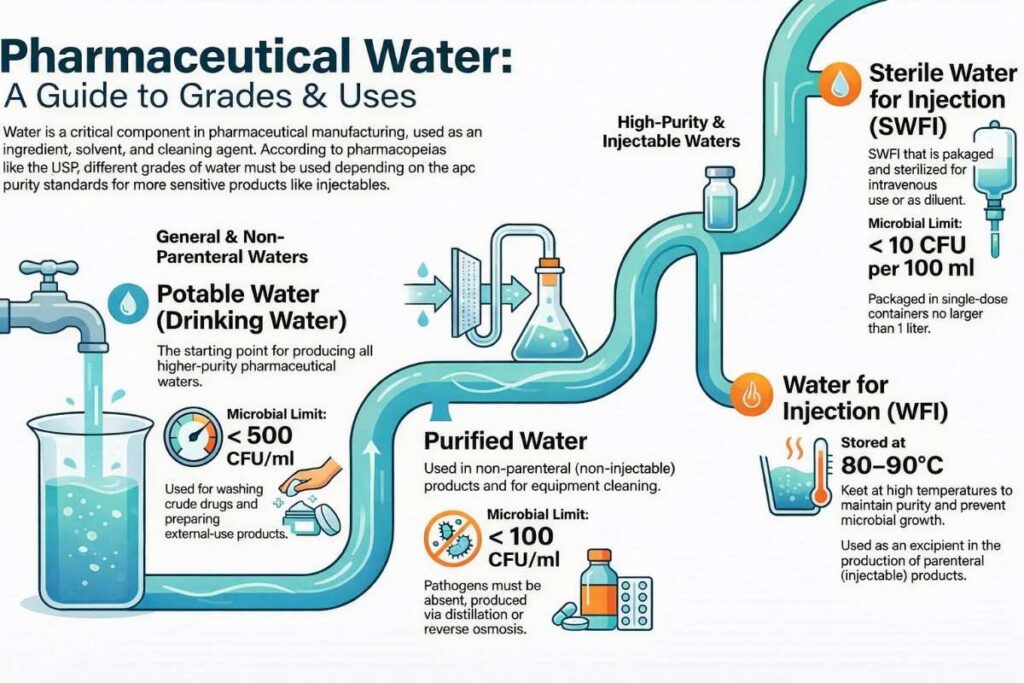
1. Potable Water (Drinking Water)
Description:
Water supplied from municipal sources that meets drinking water standards.
Uses:
-
Initial washing of equipment and containers
-
Feed water for further purification systems
Preparation Technique:
-
Filtration
-
Chlorination or ozonation (municipal treatment)
-
Not suitable for direct pharmaceutical use
2. Purified Water (PW)
Description:
Water obtained by purification of potable water and meeting pharmacopeial standards for chemical purity.
Uses:
-
Preparation of non-sterile products (oral liquids, syrups, tablets)
-
Cleaning of equipment and utensils
-
Preparation of reagents
Preparation Techniques:
-
Reverse Osmosis (RO)
-
Deionization (DI)
-
Distillation
-
Ultrafiltration (often combined with RO)
Key Quality Attributes:
-
Low ionic content
-
Controlled microbial levels
-
No added disinfectants
3. Water for Injection (WFI)
Description:
High-purity water with strict limits on endotoxins.
Uses:
-
Manufacture of parenteral products
-
Final rinse of sterile equipment
-
Preparation of sterile solutions before sterilization
Preparation Techniques:
-
Distillation (multi-effect or vapor compression)
-
Membrane systems (RO + ultrafiltration, where permitted)
Key Quality Attributes:
-
Very low microbial load
-
Endotoxin level ≤ 0.25 EU/mL
4. Sterile Water for Injection (SWFI)
Description:
WFI that has been sterilized and packaged in sterile containers.
Uses:
-
Diluent for injectable drugs
-
Reconstitution of sterile powders
Preparation Techniques:
-
Produced from WFI
-
Sterilized by moist heat
-
Aseptically filled and sealed
Note:
Contains no preservatives.
5. Sterile Water for Irrigation
Description:
Sterile water intended for external use in large volumes.
Uses:
-
Surgical irrigation
-
Wound cleaning
Preparation Techniques:
-
Prepared from Purified Water or WFI
-
Sterilized and packaged in large containers
Note:
Not suitable for injection.
6. Sterile Water for Inhalation
Description:
Sterile, pyrogen-controlled water.
Uses:
-
Inhalation therapy
-
Nebulizer solutions
Preparation Techniques:
-
Prepared from Purified Water
-
Sterilized and packaged aseptically
7. Bacteriostatic Water for Injection
Description:
Sterile water containing a preservative.
Uses:
-
Multiple-dose injectable preparations
Preparation Techniques:
-
WFI + antimicrobial agent (e.g., benzyl alcohol)
-
Sterilization and aseptic filling
Note:
Not recommended for neonates.
8. Water for Hemodialysis
Description:
Highly purified water used in dialysis treatment.
Uses:
-
Preparation of dialysis fluids
Preparation Techniques:
-
RO
-
Deionization
-
Carbon filtration
-
UV treatment
Conclusion
Pharmaceutical water systems are designed based on intended use, regulatory requirements, and patient safety. Understanding the different types of water, their applications, and preparation methods is essential for pharmaceutical manufacturing, quality assurance, and compliance.